The ROV Global Explorer reaches bottom at around 9:01 am, nearly 3.5 kilometers deep in the Gulf of Mexico. The 1.5-ton machine flies nimbly through a shallow valley before rising up and over a peak. As we descend the other side, we hold our breath in anticipation. Will we see our target? Out of the darkness, its silhouette emerges—a deep-sea brine pool. Here, lies a small pond of dense water, nearly 7 times more saline than the surrounding ocean. This small pond of dense water is nearly seven times more saline than the surrounding ocean. Its density and salinity keep it separate from the ocean above, much like oil and vinegar. It’s otherworldliness is striking. Cold seeps, where fluids rich in hydrogen sulfide, methane, and other hydrocarbons seep out, dot the seascape of the deep Gulf of Mexico, forming often unique features like brine pools.
We fly along the shoreline and notice dozens of dead urchins. The brine’s high density, which prevents it from mixing with the ocean above, also means that oxygen is not mixed in, creating an anoxic deathtrap for unsuspecting respiring organisms. As we continue to explore, a small, semi-submerged mound in the middle of the brine pool begins to pulse and vent. As we maneuver the Global Explorer closer, we see hundreds of cavities all venting fluid. We take a sample of mud around one of these pits, in hopes of capturing its invertebrate builder. Later on board the vessel, we examine the sample and discover a type of worm, a sipunculid, half the size of hot-dog with the bright green, gold, and purple colors. Students in my research group begin almost immediately referring to it as the Mardi Gras Worm. How can this worm survive in such a toxic place?
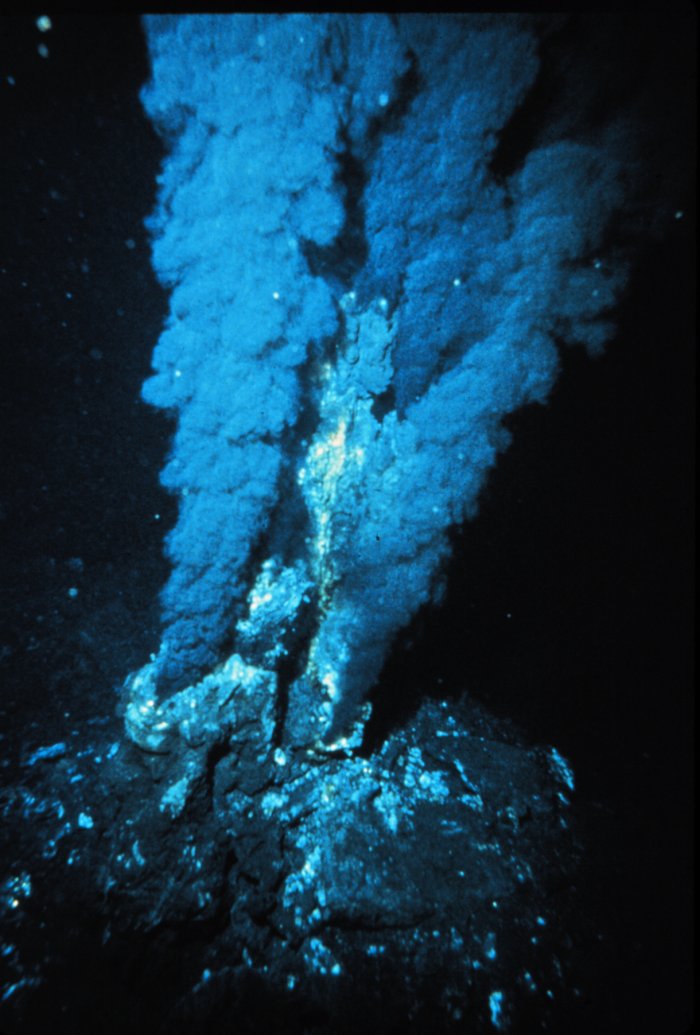
Both cold seeps and their chemical and geological relatives, hydrothermal vents, are rich in reduced chemicals, particularly hydrogen sulfide. These chemicals are toxic. Sulfide and oxygen don’t naturally coexist for long because they react spontaneously and rather aggressively. However, this sulfide also provides a unique energy source, by willingly donating electrons, to chemosynthetic microbial live. These microbes use this to fix carbon and produce food. This makes vents and seeps different than the rest of the deep in being largely independent of sunlight and the photic zone above. Many organisms in these environments either feed on free-living bacteria or form symbiotic relationships with chemosynthetic bacteria to obtain their food. This makes life challenging for both microbes and the animals that depend on them, as they need both sulfide and oxygen.
Despite these harsh conditions, these unique ecosystems flourish, showcasing the remarkable adaptability of life in extreme environments. The hydrothermal vent worms, the Alvinellids, build tubes projecting from chimney walls, giving their gills access to oxygen-rich water. The other end of the tube can then access the vent fluids rich in hydrogen suflide. Mobile predators like the crab, Bythograea thermydron can move between areas with and without oxygen. Ice worms, so names because they are found on methane ice in the cold seeps of the Gulf of Mexico, circulate oxygenated water around itself using its bristle-bearing appendages. The adaptations extend well into the biochemical level. The massive vent worm, Riftia pachyptila and the Alvnellids contain hemoglobins, giving them bright red colors, which bind insistently to oxygen.

But once dealing with oxygen issue, how do these species survive the toxic sulfide and heavy metal soup of vents and seeps. One way means of survival for these organisms is prevent sulfide from even reaching sensitive tissues. Creating sulfide barriers may mean creating thick tubes or cuticles to prevent the skin from encountering suflide. For example, the Pompeii worm, Alvinella pompejana, at hydrothermal vents secretes proteinaceous tube that it shares with bacteria. Animals that inhabit chemosynthetic habitats also often possess a specialized blood protein that binds to sulfide—forever—preventing it from mucking up the business of respiring oxygen. At hydrothermal vents, metals reach such high concentrations they precipitate out of water. These heavy metal solids form the impressive chimney structures of vents and even coat the tubeworm tubes and the shells of snails and clams. Chemosynthetic organism have ways of dealing with this too. Special metal-binding proteins, called metallothioneins, grab toxic metals and even grouping together to form little bodies or particles distinct from the rest of the cell. These consolidated and enclosed heavy metals then stay out of the way and do not gum of the cellular works.

Overall, the exploration of these deep-sea ecosystems reveals the astonishing resilience and adaptability of life in extreme conditions, offering valuable insights into the limits of biological diversity and the potential for life beyond Earth.