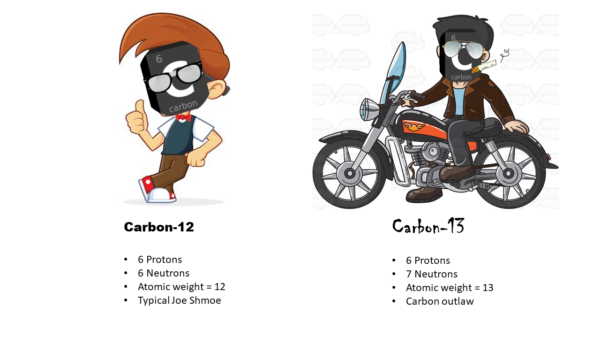
Remember all the details about the periodic table from high school chemistry? Yeah, me neither. Don’t worry – we will get through this together. Let’s focus on carbon. Carbon is the building-block of all organic (i.e., living) things, so it’s kind of a big deal. All the other elements are a little bit jealous. Okay, so on the square for carbon in the periodic table, there’s a giant C – for carbon… okay got it. But there’s all these mysterious numbers around the big C. WHAT DO THEY MEAN!!!??? SOMEONE PLEASE SEND HELP!!!! I’m remembering why I never really liked Chemistry class…….. Breathing… but seriously, I guess it’s not that hard. The first number is a “6”, and this is its “atomic number.” It corresponds to how many protons and how many neutrons it has. You add protons + neutrons to get the “atomic weight” of the element, in this case 12. Okay, this is fine, simple math… but not all carbons follow these rules (UGH).

These bada$$ carbons are “isotopes”, sort of like fraternal twins (or triplets/quadruplets) where one is blazing their own path. One of the twins is your regular Joe Shmoe who follows the rules and does everything by the book. These are the ones shown in the periodic table. The other twin in each set has the same number of protons as its boring twin, but it doesn’t follow the rules about how many neutrons they are supposed to have. They’re greedy little thieves. So, they are technically the same element, but they end up weighing different. For instance, Carbon-13 has his regular six protons like its brother, but it has a whopping seven neutrons because it just haaaad to go and be extra cool.
Almost every element has some number of isotopes/twins, except weird ones like Thulium and Holmium – but who even are those guys? Now, the wrong-number-of-neutrons outlaw twin can either be “stable” or “unstable”. It’s like the difference between the cool guy in class and the guy who is so “cool” that he ends up expelled from school. The stable ones are functional in society – in this case meaning they occur in nature without a problem. The unstable ones are completely dysfunctional and over time try to turn back into their more stable twins by shedding neutrons. It’s kind of like they just went too neutron-crazy, got a little wild, and now they’re all bloated and not having a good time.
Knowing about these different carbons is important because stable isotopes can help reveal food webs. Naturally occurring carbon consists of both the normal carbon and its bad boy twin. We have a method that allows us to measure the ratio between the outlaw and the normal (we call this ratio the isotopic ratio). By measuring the carbon isotopic ratio of an animal, we can answer questions like what did this animal eat, what level consumer are they, and even what kind of eater are they (suspension feeder, predator, etc). This is especially important in my work because I want to understand how carbon from land makes it into the deep-sea food web. When I drop a big hunk of land carbon in the form of an alligator or a wood log (wood fall), I first measure the ratio of good boy to bad boy carbon in that particular hunk of food. I also collect samples of the sediments around where I drop the food and measure the ratio of carbons in that sample too. Then, after letting the food stay on the bottom of the ocean for a while, I can take animals directly off of it and take similar animals far away from the it. When I measure the ratio of carbons in these animals, I can compare them to the ratio of the two food sources I measured and can understand which food source the animals are using.

The reason this all works is because of the saying “you are what you eat.” Turns out that is actually true! We know how much the good boy to bad boy carbon ratio should change from a food item to its consumer. This is especially helpful as we begin moving up the food web, because we can start to see who is eating whom – and this is something not yet well understood in the deep sea.
Share the post "You are what you eat! Using bad boy carbons to understand food webs"