Part II: How toxic are dispersants?
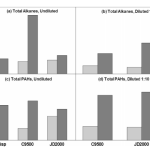
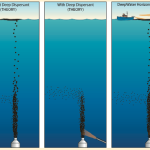
![]() This, I suppose, is the million-dollar question. The EPA has continually insisted that the actual dispersants is less toxic than dispersed oil. Ok, oil is full of some pretty nasty compounds, and the studies do in fact back up this claim. If you spray Corexit on some shrimp, and then spray crude oil on another group of shrimp, the ones exposed to the oil will fare much worse. But the EPA’s statement is actually pretty sneaky, because what most studies also say is that chemically dispersed oil (dispersant-crude oil combinations) is more toxic than physically-dispersed oil (e.g. oil scattered by wave action). Chemically dispersed oil results in a 5 to 10-fold increase in highly toxic polyaromatic hydrocarabons (PAHs) within the water column, greatly increasing the likelihood that marine organisms to come into contact with these compounds.
This, I suppose, is the million-dollar question. The EPA has continually insisted that the actual dispersants is less toxic than dispersed oil. Ok, oil is full of some pretty nasty compounds, and the studies do in fact back up this claim. If you spray Corexit on some shrimp, and then spray crude oil on another group of shrimp, the ones exposed to the oil will fare much worse. But the EPA’s statement is actually pretty sneaky, because what most studies also say is that chemically dispersed oil (dispersant-crude oil combinations) is more toxic than physically-dispersed oil (e.g. oil scattered by wave action). Chemically dispersed oil results in a 5 to 10-fold increase in highly toxic polyaromatic hydrocarabons (PAHs) within the water column, greatly increasing the likelihood that marine organisms to come into contact with these compounds.
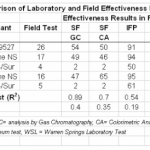
Of the recent toxicity studies, most researchers (about 75%) found that chemically-dispersed oil was more toxic than physically dispersed oil. (Fingas, 2008)
The sensitivity of marine taxa varies between species and life stages, but generally larvae and embryos are much more susceptible to dispersants and dispersed oil than adults. Studies such as Khan and Payne (2005) found that minute oil droplets in can physically damage fish gills and result in noticeable lesions. There is also a complete lack of long-term studies.
The U.S. National Academy of Science report on dispersants…notes several times that there is insufficient understanding of the fate of dispersed oil in aquatic systems, particularly interaction with sediment particles and subsequent effects on the biotic components. The relative important [sic] of different routes of exposure, that is, the uptake and associated toxicity of oil as dissolved components compared to oil droplets and also as mineral particle-associated droplets is poorly understood. Many exposure models and studies do not consider these differences either. (Fingas, 2008)
Oh, and you remember those PAHs? Well, in the presence of natural sunlight (e.g. the top 200m of the water column), they become 12 to 50,000 times more toxic. So, as chemical dispersion spreads the PAHs around, sunlight is supercharging their potency in shallower depths.
References:
Fingas, M.F. (2008). A Review of Literature Related to Oil Spill Dispersants 1997-2008 Prince William Sound Regional Citizens’ Advisory Council (PWSRCAC) Report
Khan RA, & Payne JF (2005). Influence of a crude oil dispersant, Corexit 9527, and dispersed oil on capelin (Mallotus villosus), Atlantic cod (Gadus morhua), longhorn sculpin (Myoxocephalus octodecemspinosus), and cunner (Tautogolabrus adspersus). Bulletin of environmental contamination and toxicology, 75 (1), 50-6 PMID: 16228872


I don’t know whether you’re looking into this in another installment, but I seem to remember that part of the problem was that Corexit was a patented dispersant product, and therefore the components/chemical structure was federally protected from release to the (scientific) public. Has this been addressed? It would seem to me that only by knowing the original chemical signature could we fully assess the pathways of it and its byproducts through the various GOM ecosystems.